- [ 메디채널 김갑성 기자 ] Robust revenue growth and substantial profit improvement
- Continued exceptional executions under a clear roadmap of dual-driven growth and global innovation
SAN FRANCISCO and SUZHOU, China, Aug. 27, 2025 -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, cardiovascular and metabolic, autoimmune, ophthalmology and other major diseases, announces its 2025 interim results and major business updates.
Dr. Michael Yu, Founder, Chairman of the Board and CEO of Innovent, stated: "We delivered an outstanding performance in the first half of 2025, driven by the comprehensive acceleration of our dual-engine growth model and global innovation strategy. Supported by our oncology leadership and successful commercial launch across our general biomedicine portfolio, we have expanded our product portfolio to 16 drugs and achieved a notable improvement in revenue and profitability, maintaining strong business momentum.
On the innovation front, several core pipeline candidates with global potential achieved key proof-of-concept data, and are entering global registration trials. This demonstrates the depth of Innovent's innovation capabilities and lays a solid foundation for globalization.
We firmly believe that a clear strategic vision combined with excellent execution will continue to drive Innovent's growth. Looking ahead, we will further consolidate our leadership in oncology, continue exploring and implementing diversified commercialization strategies across our general biomedicine product portfolio, and accelerate the global development of our next-generation innovative pipeline. Anchored by our strategic goals of achieving RMB 20 billion in product revenue by 2027 and advancing five pipeline programs into global Phase 3 trials by 2030, Innovent will continue striving to become a world-class biopharmaceutical company."
Dual-driven revenue growth with execution excellence
[ 메디채널 김갑성 기자 ] Robust revenue growth and substantial profit improvement[1]
- Total revenue: RMB 5.95 billion, up 50.6% year-on-year
- Product revenue: RMB 5.23 billion, up 37.3% year-on-year
- Net profit: RMB 1.21 billion; EBITDA: RMB 1.41 billion
- Gross margin: 86.8%, up 2.7 percentage points year-on-year
- Selling and administrative expenses ratio: 44.2%, down 7.9 percentage points year-on-year
- R&D investment: RMB 903 million
- Cash on hand: approximately USD 2 billion[2]
Dual-engine growth supported by comprehensive and diversified product portfolio
- Expansion of oncology product portfolio with three new launches
- Dovbleron® (taletrectinib) : Potentially best-in-class ROS1 inhibitor
- Limertinib: Third-generation EGFR TKI
- Jaypirca®(pirtobrutinib) : First non-covalent BTK inhibitor
- Strengthening general biomedicine portfolio with innovative pipeline
- SINTBILO® (tafolecimab injection): First PCSK-9 inhibitor successfully included in the NRDL, received approval by Macau ISAF in May 2025
- SYCUME® (teprotumumab N01 injection): First approved anti-IGF-1R monoclonal antibody, ending a 70-year drought of no new treatment options for thyroid eye disease (TED) in China
- Mazdutide (GCG/GLP-1) : Globally first and only GCG/GLP-1 dual receptor agonist for weight management, with another NDA for glycemic control of T2D adults under NMPA review
Innovative commercial strategy with multi-channel coverage

- Actively responding to the national "Year of Weight Management": Participating in the development of the weight-loss industry ecosystem, promoting the concept of scientific weight management, and advancing obesity prevention and chronic disease management
- Deepening hospital presence and academic influence: Strengthening academic coverage in public hospitals, with the GLORY-1 study published in The New England Journal of Medicine, significantly enhancing academic influence
- Innovative multi-channel coverage: Leveraging a comprehensive sales team of over 1,000 people, integrating public hospitals, retail pharmacies, online medical platforms, and private clinic networks to facilitate convenient access to medications for chronic disease patients
- Emphasizing disease education and patient management: Integrating digital tools and professional activities to establish a patient-centric disease management system, enhancing chronic disease education and patient adherence
Enhancing brand strength through the establishment of a lifecycle management system
- TYVYT®: NDAs for renal and colorectal cancer are under regulatory review; Phase 3 study of neoadjuvant treatment in lung cancer is underway
- SYCUME® (teprotumumab N01 injection): new Phase 3 studies in plan for inactive TED and head-to-head comparison with steroid therapy in TED
- Mazdutide (GCG/GLP-1): second NDA for glycemic control of T2D adults is under regulatory review, alongside with two new Phase 3 studies for obstructive sleep apnea (OSA) and obesity with metabolic dysfunction-associated fatty liver disease (MAFLD, head-to-head with semaglutide 2.4mg). Additional PoC studies are ongoing for adolescent obesity, metabolic dysfunction-associated steatohepatitis (MASH), heart failure with preserved ejection fraction (HFpEF), etc.
- Picankibart (IL-23p19): a potent and long-acting IL-23p19 for moderate-to-severe plaque psoriasis pending NMPA approval; Phase 3 studies are ongoing for treatment withdrawal maintenance and psoriasis with prior inadequate response to IL-17 biologics
Emerging value from globalization strategy, prioritizing novel pipeline's global R&D
Flagship pipeline showcased at top-tier conferences, entering global registration studies
- IBI363 (PD-1/IL-2α-bias): Unleashing potential as a next-generation IO therapy with global first-in-class design
- Three oral presentations at ASCO highlighted breakthrough PoC data across difficult-to-treat tumors such as IO resistant cold tumors and low PD-L1 expression, demonstrating the unique advantages of dual immune activation and long-term survival benefits
- Three pivotal studies are underway or in plan: the first global Phase 3 study is initiating in China and the U.S. to address unmet needs in IO-resistant squamous NSCLC; the first pivotal Phase 2 study in melanoma was initiated, challenging Keytruda in first-line setting; and the first Phase 3 study in late-line colorectal cancer is about to initiate
- Continuous exploration in first-line settings and more cancer types, including first-line lung cancer, first-line colorectal cancer, neoadjuvant lung cancer, EGFR-mutated lung cancer, platinum-resistant ovarian cancer, and more
- BI343 (CLDN18.2 ADC): two registration trials in GC and PDAC; globally the first ADC to enter a Phase 3 trial in PDAC
- First MRCT Phase 3 study of GC has been initiated in China and Japan
- First Phase 3 study of PDAC has been initiated
- Global MRCT of PDAC is in plan
- NMPA CDE Breakthrough Therapy Designation (BTD) and FDA Fast-track Designation (FTD) granted
- Over 10 next-generation programs advancing into global development
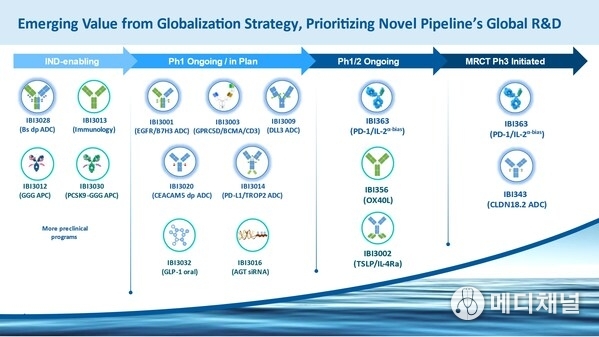
- Oncology: IBI3009 (DLL3 ADC), IBI3001 (EGFR/B7H3 ADC), IBI3003 (GPRC5D/BCMA/CD3), IBI3005 (EGFR/HER3 ADC), IBI3014 (PD-L1/TROP2 ADC), IBI3020 (CEACAM5 dual-payload ADC), etc
- CVM: IBI3016 (AGT siRNA), IBI3032 (oral GLP-1), etc
- Autoimmune: IBI356 (OX40L), IBI3002 (TSLP/ IL-4Rα), etc
Hybrid models to accelerate innovation
- IBI3009 (DLL3 ADC): global rights granted to Roche, to benefit SCLC patients worldwide
Enhance global presence with broader market access
- Six products (TYVYT®, BYVASDA®, Pemazyre®, Dupert®, SINTBILO®, SYCUME®) have been launched in markets including Hong Kong, Macau, Taiwan, and Southeast Asia
- More launches planned Brazil, Mexico, Columbia, India, etc
Research innovation showcased at medical conferences
- Oncology pipeline: AACR, ASCO, Nature Medicine
- General biomedicine pipeline: ADA, NEJM
Facilities and manufacturing capacity adhering to high-quality standards
- 7,500 employees globally
- Global R&D centers located in the San Francisco Bay Area, Shanghai, and Suzhou
- To date, Innovent has a total of 140,000L of operational capacity, accounting for 20% of China's total biopharmaceutical production capacity
- First manufacturing site: 60,000L antibody and ADC production capacity in operation, ensuring high-quality supply
- Second manufacturing site: first phase of 80,000L antibody production capacity in operation, for global supply and CDMO operations
Committed to responsible business practices and enhancing ESG management practices
- Over 3,000 new oncology patients begin treatment with Innovent-developed therapeutics each day, and to date, more than 5 million patients have benefited from Innovent's innovative drugs
- Innovent has been graded 'AAA' rating in MSCI ESG rankings, positioning us at the forefront of the biotechnology industry
- We have supported over 200,000 patients through our dedicated patient assistance programs, with drug donations totaling RMB 3.6 billion
- Our commitment to medical philanthropy has been recognized through prestigious awards, including the "Medical Philanthropy Promoter" title and designation as a "China Philanthropic Enterprise"
- Provided over 2,200 job opportunities for recent graduates
- To date, Innovent Biologics has contributed more than RMB 6 billion in taxes and fees
About Innovent Biologics
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 16 products in the market. It has 2 new drug applications under regulatory review, 4 assets in Phase III or pivotal clinical trials and 15 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Eli Lilly, Sanofi, Incyte, LG Chem and MD Anderson Cancer Center.
Guided by the motto, "Start with Integrity, Succeed through Action," Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit www.innoventbio.com, or follow Innovent on Facebook and LinkedIn.
Statement:
(1)Innovent does not recommend the use of any unapproved drug (s)/indication (s).
(2)Ramucirumab (Cyramza),Selpercatinib (Retsevmo) and Jaypirca (pirtobrutinib) were developed by Eli Lilly and Company.
Forward-Looking Statements
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent, are intended to identify certain of such forward-looking statements. Innovent does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of Innovent with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond Innovent's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, Innovent's competitive environment and political, economic, legal and social conditions.
Innovent, the Directors and the employees of Innovent assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialize or turn out to be incorrect.
[1] Note:The financial numbers mentioned above, unless stated, were based on non-IFRS measure. Detailed disclosure can be found at the Company's 2025 interim results announcement.
|
[2] Note:cutoff July 31st, 2025.
|
