-

Healgen Scientific Spotlights the Rapid Check® COVID-19/Flu A&B Antigen Test to Help Families Stay Prepared for the Respiratory Virus Season
HOUSTON, Nov. 27, 2025 -- As the holiday season approaches, gatherings among family and friends are expected to increase the risk of respiratory virus transmission, including COVID-19 and influenza. In response, Healgen® Scientific highlights the Rapid Check® COVID-19/Flu A&B Antigen Test, a 3-in-1 at-home rapid test designed to help users quickly distinguish between COVID-19 and common flu viruses. According to the U.S. Centers for Disease Control and Prevention (CDC), the upcoming 2025–2026 respiratory disease season is expected to bring a comparable level of hosp
- 김갑성 기자
- 2025-11-27 00:00
-
TCI Biotech Inaugurates Advanced Performance Nutrition Research Center, Expands Into Brain Science to Accelerate Next-Generation Health Innovation
SALT LAKE CITY, Nov. 27, 2025 -- TCI Biotech (TCI Co., Ltd.), a global CDMO+ specializing in functional foods, nutraceuticals, and beauty supplementation, today announced the launch of the TCI Advanced Performance Nutrition Research Center, a new innovation hub focused on high-performance nutrition, cognitive wellness, healthy aging, and sports performance. This milestone marks TCI's strategic entry into the fast-growing field of brain science, supported by new research programs in regenerative medicine, cellular biology, and neuro-supportive ingredient technologies. The opening of the
- 김갑성 기자
- 2025-11-27 00:00
-

China Cycle 2026: Building An Innovation Driven, Intelligence-led Future
SHANGHAI, Nov. 26, 2025 -- The 34th China International Bicycle Fair (China Cycle 2026) is expected to unveil the grand exhibition from May 5 to 8, 2026 at the Shanghai New International Expo Center (SNIEC), themed "Innovation-Driven, Intelligence-Led Future." Building on three decades of expertise and innovation, China Cycle has become a pivotal hub shaping global trends in the bicycle and e-bike industries and a leading professional platform with deep industry influence. In 2024, China's total bicycle production reached nearly 99.54 million, the imports of race bicy
- 김갑성 기자
- 2025-11-26 22:10
-

2026년 상하이 국제 자전거 박람회: 혁신을 원동력 삼은 첨단 기술 중심의 미래 건설
[ 메디채널 김갑성 기자 ] 상하이 2025년 11월 26일 -- 차이나 사이클(China Cycle)로도 알려진 제34회 상하이 국제 자전거 박람회(China International Bicycle Fair)가 '혁신을 원동력 삼은 첨단 기술 중심의 미래(Innovation-Driven, Intelligence-Led Future)'를 주제로 2026년 5월 5일부터 8일까지 상하이 신국제 엑스포센터(Shanghai New International Expo Center, SNIEC)에서 열릴 예정이다. 이나 사이클은 30년간 전문성과 혁신을 축적하면서 세계 자전거 및 전기 자전거 산업의 유행을 이끄는 구심점이자 업계에 막대한 영향을 미치는 세계 유수의 행사로 자리매김했다. 2024년 중국의 총 자전거 생산량은 약 9954만 대에 달했다. 경기용 자전거 수입량은 전년 대비 12.5% 증가했으며, 고급 전기 자전거가 지난해 전체 시장에서 차지하는 비중은 19.1%였다. 전략적 교두보 역할을 하는 상하이 국제 자전거 박람회는 해외 기업들이 중국의 광범위한 시장의 문을 직접 두드린 후 중국 소비자 심리에 대한 의미심장한
- 김갑성 기자
- 2025-11-26 22:10
-

Celltrion announces publication of post-hoc analysis of LIBERTY-CD study of ZYMFENTRA® (infliximab-dyyb), indicating consistent efficacy across disease location, including the terminal ileum in Clinical Gastroenterology and Hepatology journal
Findings from the post-hoc analysis of LIBERTY-CD study indicates efficacy of ZYMFENTRA® (subcutaneous infliximab) regardless of disease location, consistent across ileum-dominant and colon-dominant Crohn's disease (CD) Findings support the therapeutic potential of ZYMFENTRA in addressing persistent treatment gaps especially in ileum-dominant CD INCHEON, South Korea, Nov. 26, 2025 -- Celltrion, Inc. today announced that a post-hoc analysis of the LIBERTY-CD study, which showed that the efficacy of ZYMFENTRA® (infliximab-dyyb) is consistent regardless of disease l
- 김갑성 기자
- 2025-11-26 14:48
-

Vital Signs highlights how researchers are "cracking the code on the genome" to harness the biomedical superpowers of hibernating animals
HONG KONG, Nov. 26, 2025 -- In a groundbreaking new episode, CNN's long-running series Vital Signs delves into the cutting-edge science of hibernation, revealing how the secrets of the animal kingdom could unlock revolutionary advancements in human health, from fighting cancer and heart disease to facilitating space travel. This November, CNN's Chief Medical Correspondent, Dr. Sanjay Gupta, takes viewers on a global journey to meet the extraordinary individuals leading the charge in understanding hibernation in mammals and potentially inducing human torpor one day. CNN
- 김갑성 기자
- 2025-11-26 14:44
-

Korea Innovation Foundation selects 2 Innovative bio health companies, LEEBIO, and Airlab for Global Technology Commercialization Support Program (North America)
DAEJEON, South Korea, Nov. 26, 2025 -- Korea Innovation Foundation (INNOPOLIS), currently operating the 2025 Global Technology Commercialization Support Program (North America), announces that 2 innovative companies, LEEBIO, and Airlab are chosen for the support program. This initiative selects innovative technology companies in the fields of machinery, energy, AI/IoT and bio health. The program provides comprehensive consulting support to help these companies apply their technologies and products on a trial basis to local facilities or projects operated by overseas companies and insti
- 김갑성 기자
- 2025-11-26 13:45
-
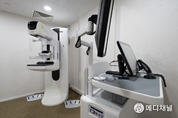
Chiron Medical Advances Women's Health with Expanded "1:1 Breast Health Empowerment Charity Campaign"
[ 메디채널 김갑성 기자 ] 300 Free 3D Mammogram Screenings Donated to Community Women, Championing Self-Care and Compassionate Support HONG KONG, Nov. 26, 2025 -- Building on its commitment to accessible healthcare, Chiron Medical Group (Referred as "Chiron") launched the "1:1 Breast Health Empowerment Charity Campaign" earlier this year, delivering 105 free 3D mammogram screenings and personalized medical consultations to underprivileged women. Through a series of six interactive breast health workshops, the initiative successfully engaged numerous low-income participants,
- 김갑성 기자
- 2025-11-26 13:03
-
The Unseen Itch: Over Half of Singaporeans With Eczema Struggle With Sleep Loss, Shame and Social Withdrawal Due to Itch
[ 메디채널 김갑성 기자 ] A recently concluded survey by Medical Channel Asia sheds light on the debilitating effects of constant itch caused by eczema. SINGAPORE, Nov. 25, 2025 -- A new ground-up survey "The Unseen Itch: How Eczema Disrupts Daily Life in Singapore" by Medical Channel Asia has uncovered a stark and often overlooked reality about itch. For people living with atopic dermatitis, or eczema, itch is not a minor inconvenience – it is a daily, debilitating force that silently dictates how they sleep, work, socialise, and even how they see themselves. The init
- 김갑성 기자
- 2025-11-26 10:33
-

Precious Hospital Sdn. Bhd. to Launch Cancer Centre and Hospital in Johor Bahru
[ 메디채널 김갑성 기자 ] First cyclotron outside Klang Valley to anchor Malaysia's advanced cancer care ecosystem KEY-HIGHLIGTHS RM500 million investment in advanced cancer care infrastructure State-of-the-art diagnostic and treatment facilities, including a nuclear medicine centre First cyclotron outside the Klang Valley enabling local radioisotope production for cancer detection and therapy Located in Larkin Sentral within the Johor–Singapore Special Economic Zone (JS-SEZ) Phase One projected to create around 1,000 direct and indirect jobs JOHOR BAHRU, Malaysia and
- 김갑성 기자
- 2025-11-26 10:00
- 1포리프 굿마팻 (누바디 굿바이 마이팻) 다이어트 건강기능식품 출시
- 22021년 식품분석전문가 1급/2급 검정자격시험 안내
- 3투썸플레이스, 초콜릿 애프리콧 무스 출시
- 4만성질환자들의 유료독감백신접종에 정책적인 배려가 필요하다
- 5아임웹, CRM 기능 출시… 고객 행동 데이터 기반 마케팅 지원
- 6하만카돈, AURA STUDIO 4 블루투스 스피커 출시
- 7대구오페라하우스 - 20주년 기념 골든 보이스 시리즈Ⅲ, 테너 콘서트
- 8대한약물영양의학회 2020년 추계학술대회
- 92021년 대학생기자단 모집
- 10대한영양제처방학회 춘계학술대회 성황리에 마쳐
2026-01-01_THU
- 하이얼, 파리 생제르맹과 손잡고 전 세계 사용자에게 챔피언 경험 선사 20:56
- Haier Teams Up with Paris Saint-Germain to Create Champion Experiences for Global Users 14:30
- Rising Through Challenge: Living Phoenix's 2026 New Year Message POGMENT Biomimetic Collagen 12:36
- 홍보 영상 '민항과 함께, 미래를 향한 승리' 공개 - Xinhua Silk Road: 전 세계 온라인 공개 10:49
- iQIYI LAND Yangzhou to Open on February 8, Marking New Chapter in China's Immersive Entertainment Scene 10:00
- OG Tech Debuts 'War Room' Series, Targets Q1 2026 for Global AI Action Center 10:00
- Hecto Media、AI搭載の多言語Kカルチャープラットフォーム「K-snapp」を発表 06:54









