-

Novel Anti-Fibrotic Drug AK3280 Cleared by FDA to Initiate Phase 2 Proof-of-Concept Clinical Trial in IPF
SHANGHAI, Feb. 12, 2026 -- Shanghai Ark Biopharmaceutical Co., Ltd. ("ArkBio") today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for AK3280, a novel anti-fibrotic therapy for the treatment of idiopathic pulmonary fibrosis (IPF). This clearance enables ArkBio to initiate a Phase 2 proof-of-concept (PoC) clinical trial of AK3280 in the United States. The Phase 2 study is a multi-center, randomized, partially double-blind, placebo- and active-controlled trial designed to evaluate the
- 김갑성 기자
- 2026-02-12 12:18
-
WuXi Vaccines Receives Brazil's ANVISA GMP Certification
SUZHOU, China, Feb. 12, 2026 -- WuXi Vaccines, a wholly-owned subsidiary of WuXi Biologics, dedicated to vaccine Contract Development and Manufacturing Organization (CDMO), announced today that it has received Good Manufacturing Practices (GMP) certification for its fill and finish facility (DP17) in Suzhou from Brazil's Agência Nacional de Vigilância Sanitária (ANVISA). The facility is providing integrated manufacturing services for Instituto Butantan's dengue vaccine (Butantan-DV) production project. Following comprehensive on-site inspections of DP17, a commercial
- 김갑성 기자
- 2026-02-12 11:48
-
Big Tree Cloud Holdings Limited Announces Update Regarding Previously Announced Reverse Share Split
SHENZHEN, China, Feb. 12, 2026 -- Big Tree Cloud Holdings Limited (the "Company") (NASDAQ: DSY) today announced that the previously disclosed reverse share split and related corporate actions, including the change in par value, reclassification, and CUSIP number change, will not become effective on February 12, 2026, as previously anticipated. The renewed effective date of the reverse share split and related corporate actions will be announced at a later time. The Company intends to issue a further press release once the new effective date has been determined. About Bi
- 황정호 기자
- 2026-02-12 10:55
-

AXA's Title-Sponsored "33rd Green Power Hike" Successfully Completes
[ 메디채널 황정호 기자 ] Jointly promoting "Leave-No-Trace" hiking and environmental conservation for a greener future HONG KONG, Feb. 12, 2026 -- The 33rd Green Power Hike, organised by Green Power and title-sponsored by AXA Hong Kong and Macau ("AXA"), successfully took place on 31 January. The event drew 3,000 participants who competed across the 10km, 25km and 50km categories. This year also marked the introduction of the inaugural "School Cup," alongside six "Corporate Cup" competitions, further broadening the event's reach and engagement. As one o
- 황정호 기자
- 2026-02-12 10:52
-
Australia and New Zealand's Skin Cancer Governance Gap Exposed: 40% of Organisations Never Assessed Workforce Risk Despite 84% Believing Duty of Care Applies
SYDNEY, Feb. 12, 2026 -- A comprehensive new report on workplace skin cancer screening in Australia and New Zealand has exposed a significant governance blind spot: whilst 84% of organisations believe skin checks should be part of their duty of care, 40% have never assessed their workforce's skin cancer risk, and 23% of boards don't understand UV exposure as a workplace health and safety concern. The research, drawn from MoleMap's analysis of 48,000+ workplace skin checks and a survey of 410 HR and wellbeing managers across ANZ, reveals a disconnect between awareness and action that pu
- 황정호 기자
- 2026-02-12 10:32
-

TOKYO CREATIVE SALON 2026 Program Highlights City-Wide Creativity Across Tokyo
TOKYO, Feb. 12, 2026 -- TOKYO CREATIVE SALON 2026 (TCS2026) will present an expanded lineup of programs under its core concept of FUTURE VINTAGE, offering visitors a city-wide experience of contemporary creativity across Tokyo. City Wide Program At the heart of TCS2026 is the City Wide Program, which transforms Tokyo itself into a creative platform. Across nine areas—each with its own history, culture, and identity—fashion, design, art, and craft installations will unfold throughout the city. Visitors are invited to walk between districts, encountering creativity
- 김장윤(JASON KIM) 기자
- 2026-02-12 10:00
-

도쿄 크리에이티브 살롱 2026, 도쿄 전역에서 도시형 창의성 프로그램 집중 소개
도쿄 2026년 2월 12일 -- 도쿄 크리에이티브 살롱(TOKYO CREATIVE SALON 2026, 줄여서 TCS2026)이 미래 빈티지(FUTURE VINTAGE)를 핵심 콘셉트로 도쿄 전역에서 현대적 창의성을 경험할 수 있도록 확장된 프로그램 라인업을 선보인다. 시티 와이드 프로그램 TCS2026의 중심에는 도쿄 도시 전체를 창의적 플랫폼으로 전환하는 시티 와이드 프로그램(City Wide Program)이 있다. 각기 고유한 역사와 문화, 정체성을 지닌 아홉 지역에서 패션, 디자인, 예술, 공예 설치물이 도심 곳곳에 전개된다. 관람객은 지역과 지역을 직접 걸으며 일상적인 도시 공간 속에서 창의성을 마주하고, 이동과 탐색을 통해 도쿄의 다채로운 표현을 체험하게 된다. 도쿄 빈티지 패션 위크 새 공식 프로그램 도쿄 빈티지 패션 위크((Tokyo Vintage Fashion Week)가 2026년 3월 13일부터 3월 15일까지 신주쿠 스미토모 빌딩 트라이앵글 플라자(Shinjuku Sumitomo Building Triangle Plaza)에서 열린다. 약 1
- 김장윤(JASON KIM) 기자
- 2026-02-12 10:00
-

Winners in the 2026 Asia-Pacific Stevie® Awards Announced
[ 메디채널 황정호 기자 ] Winners in Thirteenth Annual Competition to Be Celebrated at 17 April Award Ceremony in Macau FAIRFAX, Va., Feb. 12, 2026 -- Winners have been announced in the thirteenth annual Asia-Pacific Stevie Awards, the only awards program to recognize innovation in the workplace throughout the entire Asia-Pacific region. The list of Gold, Silver, and Bronze Stevie Award winners is available at https://Asia.StevieAwards.com. The Stevie Awards are widely considered the world's premier business awards, conferring recognition for achievement over the past 24 years t
- 황정호 기자
- 2026-02-12 09:00
-

ADB grants PH fintech leader GCash with $30-M credit facility to expand loan access to MSMEs, women entrepreneurs, especially in high poverty areas
MANILA, Philippines, Feb. 12, 2026 -- The Asian Development Bank (ADB) and Fuse Financing Inc., the lending arm of GCash, are set to spur the growth of the Philippine micro, small, and medium enterprise (MSME) sector with a $30-million landmark loan program, a first-of-its-kind partnership in the Asean fintech landscape. "This fintech partnership is the first-of-its kind for ADB in the Asean region and marks a significant step in advancing financial inclusion in the Philippines," said Isabel Chatterton, Director General, Private Sector Operations Department of Asian D
- 황정호 기자
- 2026-02-12 08:00
-
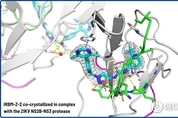
IRBM, 지카 바이러스 치료제 개발에서 획기적 성과 보고
로마, 2026년 2월 11일 -- 초기 신약 개발 연구 분야의 선도 기업 IRBM이 지카 바이러스 프로테아제(NS2B-NS3)를 표적으로 하는 신규 고효능 알로스테릭 억제제를 개발하는 중대한 과학적 돌파구를 달성했다고 발표했다. 이번 연구 결과는 국제 학술지 네이처 커뮤니케이션즈(Nature Communications, doi: 10.1038/s41467-026-68943-x)에 게재됐으며, 전임상 모델에서의 유효성을 입증함과 동시에 지카 바이러스 감염 대응을 위한 새로운 치료 접근법을 제시한다. 모기를 매개로 전파되는 지카 바이러스는 심각한 신경학적 합병증과의 연관성으로 인해 중대한 공중보건 위협으로 간주되고 있다. 현재 승인된 항바이러스제나 백신이 없는 상황에서, 새로운 치료 옵션에 대한 긴급한 수요가 지속되고 있다. IRBM 연구진은 바이러스 복제에 필수적인 핵심 효소인 NS2B-NS3 프로테아제의 기존에 규명되지 않았던 알로스테릭 부위에 결합하는 저분자 화합물을 확인했다. 해당 억제제는 생화학적•세포 기반 분석에서 프로테아제 활성을 효과적으로 억제했으며, 동물 모델에서도 유의미한 항바이러스 활성
- 김갑성 기자
- 2026-02-12 07:01
- 1포리프 굿마팻 (누바디 굿바이 마이팻) 다이어트 건강기능식품 출시
- 22021년 식품분석전문가 1급/2급 검정자격시험 안내
- 3투썸플레이스, 초콜릿 애프리콧 무스 출시
- 4하만카돈, AURA STUDIO 4 블루투스 스피커 출시
- 5아임웹, CRM 기능 출시… 고객 행동 데이터 기반 마케팅 지원
- 6대구오페라하우스 - 20주년 기념 골든 보이스 시리즈Ⅲ, 테너 콘서트
- 7만성질환자들의 유료독감백신접종에 정책적인 배려가 필요하다
- 82021년 대학생기자단 모집
- 9대한약물영양의학회 2020년 추계학술대회
- 10대한영양제처방학회 춘계학술대회 성황리에 마쳐
2026-03-02_MON
- ECR 2026: Esaote Group Introduces Breakthroughs in Ultrasound, MRI and Enterprise Imaging 19:30
- ECR 2026: 에사오테 그룹, 초음파, MRI, 엔터프라이즈 이미징 분야 혁신 기술 공개 19:30
- Galaxy AI Expands Multi-Agent Ecosystem To Give Users More Choice and Flexibility 18:45
- Samsung Puts AI-Driven Innovation Front and Centre With The Launch Of The Galaxy S26 Series & Galaxy Buds4 Series and the New Galaxy Club 18:42
- TECNO Expands AI Ecosystem at MWC 2026, Forging the "Connection of Intelligence" 17:00
- ATFX Strengthens Strategic Engagements Across Key Financial Hubs 17:00
- Shangpu Group Issues Market Position Statement Recognizing SALIAI's Patented Cellular Essence Global Pioneer 16:14








